|
|
 |
Size of Delivery Device (Minimum and Maximum Dimensions From Technical and Biological Point of View)
A single 'device', as pictured (Figure 1), is approximately 3 mm in diameter and 10 mm long and contains a 35 mg drug core payload (ca. 2.3 mm in diameter and 5.0 mm long). This payload mass (volume) has been used for convenience of handling. It is anticipated that, for commercial applications, a 'device' accommodating a 35 mg drug core would have rounded ends, as illustrated schematically in Figure 2 and be approximately 2.0 mm in diameter and 5.0 mm long.

Figure 1: Photograph of a CR 'devices' as currently produced
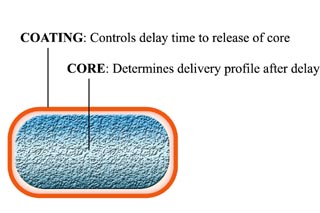
Figure 2: Schematic of CR 'device' following further process development
The maximum 'device' size would be governed by payload requirements and ethical considerations. To date the largest 'device' we have produced for testing in vivo (cattle) contained a 500 Ál fluid payload (a 'device' approximately 6 mm in diameter and 25 mm in length).
One of the independent POC trials (below) entailed administration of four (4) 'devices' (plus a 'priming' dose), each of the 'devices' being ca. 4 mm in diameter and 15 mm long, and each 'device' carrying a 200 mg 'payload'.
The table below presents data pertaining to dose 'mass' and 'device' volume for tableted (cylindrical) solid dose drug cores.
| Drug-core mass (mg) |
CRD volume |
CRD dimension (l x ° mm) |
| 5 |
7 |
1.4 x 3 |
| 35 |
50 |
2.2 x 5 |
| 50 |
70 |
2.2 x 7 |
| 100 |
140 |
2.7 x 9 |
| 250 |
360 |
3.7 x 11 |
| 500 |
715 |
4.2 x 16 |
| 1000 |
1430 |
5.0 x 21 |
Figure 3: Veterinary Implanting Equipment (larger images on click)
The means by which delay of release is achieved is independent of the 'drug dose' formulation (the 'core' of the 'device' in Figure 2).
If the drug dose contains hydrophilic materials, it will wet and disperse/dissolve rapidly, thus providing a pulsatile delivery profile. On the other hand, if the drug dose contains hydrophobic materials it will wet only slowly and slough off (erode) at a rate dependent (in general terms) on the partition coefficient, thus providing a sustained release/delivery profile. Flexibility in the release profiles achievable is because of the independence of the payload from the mechanism for control of (delay of) release of the dose(s).
main | CRD | POC | IP | Documents | Company Profile
 |
 |
||||||
|
|
|||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|